
Exposure to sublethal concentrations of antifoulant diuron reduces cholinergic activity and modulates antioxidant components in a marine polychaete
Abstract
This study aimed to investigate the potential detrimental effects of diuron, an antifoulant, on non-target benthic organisms, focusing on the marine polychaete Perinereis aibuhitensis. The marine polychaetes were exposed to sublethal concentrations of diuron (1/50, 1/20, and 1/10 of the 96 h-LC50 value) for a period of 14 days. The results showed that higher concentrations of diuron led to a significant reduction in burrowing activity and acetylcholinesterase enzyme activity in the marine polychaete. These effects indicate that diuron has cholinergic inhibitory potential and adversely affects the behavior of the polychaete by disrupting nerve impulse transmission. Additionally, higher concentrations of diuron induced oxidative stress in the marine polychaete, as evidenced by increased levels of malondialdehyde and depletion of glutathione. Interestingly, lower concentrations of diuron resulted in elevated enzymatic activities of crucial antioxidant defense components, including catalase, superoxide dismutase, glutathione reductase, and glutathione peroxidase. However, at higher concentrations of diuron, the activities of these enzymes were significantly reduced, suggesting impaired antioxidant defense mechanisms. These findings highlight that even sublethal levels of diuron can have harmful effects on the homeostasis and oxidative status of the marine polychaete. The study emphasizes the need to consider the potential impacts of the antifoulant diuron on non-target organisms, shedding light on the importance of addressing the environmental risks associated with its use.
초록
본 연구는 다모류 Perinereis aibuhitensis 대상 비표적 저서생물에 대한 방오제 diuron의 유해성을 조사하는 것을 목적으로 한다. 다모류는 14일 동안 준치사 농도 diuron(96 h-LC50 값의 1/50, 1/20 및 1/10)에 노출시켰으며, 처리 농도 중 가장 높은 농도의 diuron이 다모류 굴 파는 능력과 acetylcholinesterase 효소 활성도를 현저하게 감소시키는 것으로 나타났다. 이러한 효과는 diuron이 콜린성 저하 작용을 갖고 있으며, 신경 전달을 방해함으로 다모류 기초 행동에 악영향을 미친다는 것을 나타낸다. 또한, 높은 농도의 diuron은 다모류에서 산화 스트레스를 유발했으며, 이는 지질과산화 유발 및 glutathione고갈로 입증되었다. 낮은 농도의 diuron 은 catalase, superoxide dismutase, glutathione reductase 및 glutathione peroxidase 를 포함한 항산화 방어 관련 효소 활성도를 증가시켰으나, 높은 농도의 diuron 처리 시 이들 효소 활성도가 크게 감소하여 항산화 메커니즘이 손상되었음을 확인하였다. 이러한 발견은 준치사 수준의 diuron도 다모류의 항상성 유지에 해로운 영향을 미칠 수 있음을 시사하며, diuron이 비표적 저서생물에 미치는 잠재적 영향을 고려해야 할 필요성을 강조한다.
Keywords:
Polychaete model, Biofoulant, Cholinergic effect, Oxidative stress, Antioxidant defense키워드:
다모류 모델, 방오제, 콜린성 효과, 산화스트레스, 항산화1. Introduction
Diuron [3–(3,4–dichlorophenyl)–1,1–dimethylurea], a phenylurea herbicide, has been widely used in both agricultural and non-agricultural settings to control the growth of weeds, grass, mosses, and bushes (Giacomazzi and Cochet[2004]). Since the total ban on organotin (e.g., tributyltin) usage in antifouling compounds by the International Marine Organisation–Marine Environment Protection Committee (IMO–MEPC) (Evans et al.[2000]; Konstantinou and Albanis[2004]), it has gained popularity as an alternative to organotin-based antifoulants in antifouling paints for the inhibition of attachment of unwanted animals on ship hull (Yebra et al.[2004]). Due to its chemical characteristics, diuron exhibits high resistance to hydrolysis and photolysis (Okamura[2002]; Thomas et al.[2002]; Giacomazzi and Cochet[2004]). However, it can enter marine ecosystems through various pathways such as ship painting, hull cleaning, anchorage, spray drift, leaching, and runoff. As a result, diuron has been detected in coastal areas globally, with concentrations ranging from 12 ng L–1 to 7.8 μg L–1 ([Dahl and Blanck[1996]; Thomas[2001]; Sapozhnikova et al.[2008]; Kaonga et al.[2015]; Saleh et al.[2016]), including in South Korea where concentrations have been reported as 0.035–1.36 μg L–1 (Kim et al.[2014]). The persistence of diuron in seawater varies depending on temperature, with a half-life ranging from one month to one year (Thomas et al.[2002]). This raises concerns about its bioconcentration and bioaccumulation potential in aquatic animals. Studies have highlighted the presence of diuron in marine fish, such as those collected from the Johor Straits estuary in Malaysia, where concentrations ranging from 8–19 μg kg–1 were reported (Mukhtar et al.[2020]). These findings underscore the need to further investigate the environmental impact of diuron in aquatic organisms, emphasizing the importance of monitoring and regulating its usage to mitigate potential risks to aquatic ecosystems.
The limited information available on the detrimental effects and toxicity of diuron in aquatic animals highlights the need for further research. Previous studies have indicated that diuron can induce oxidative stress in aquatic animals, as evidenced by increased levels of reactive oxygen species (ROS) and oxidative damage (Sánchez-Muros et al.[2013]; Sánchez-Muros et al.[2014]; Behrens et al.[2016]; Velki et al.[2017]; Nam et al.[2023]). Reproductive disorders with anti-androgenic potential and genotoxicity have also been reported in certain species, such as Nile tilapia (Perissini-Lopes et al.[2016]) and oysters (Akcha et al.[2020]), respectively. However, the acute toxicity of diuron and its potential harmful effects at environmentally relevant levels on non-target animals remain poorly understood. In this study, several biomarkers were employed to assess the potential effects of diuron. Acetylcholinesterase (AChE) activity, which is widely used as an indicator of neural toxicity, was measured to assess any adverse effects on the nervous system (Nunes[2011]). Several in vivo and in vitro experiments in model systems have suggested that fluctuations in oxidative homeostasis can reduce AChE activity through direct ROS damage on AChE gene transcription, lipid damage in cell membrane structure or through a reversible oxidation process in AChE function (Schallreuter et al.[2004]; Molochkina et al.[2005]; Rico et al.[2007]; Schallreuter and Elwary[2007]; Corrêa Mde et al.[2008]). The marker malondialdehyde (MDA) was used to evaluate lipid peroxidation, a consequence of damage to polyunsaturated fatty acids in cell membranes caused by ROS (Lushchak[2011]). Additionally, antioxidant components such as glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx) were measured to assess the animals' antioxidant defense system. These components play a crucial role in the biotransformation and detoxification of intracellular ROS and free radicals, which are often increased during oxidative stress conditions (Livingstone[2001]). By utilizing these biomarkers, this study aimed to provide insights into the potential effects of diuron on biochemical responses in marine polychaetes, shedding light on its toxicity and detrimental effects.
Marine polychaetes have been widely utilized in toxicological studies of the benthic environment as sentinel species due to their small size, ability to deposit feed, ease of maintenance in the laboratory, endurance in adverse environmental conditions, sensitivity to various contaminants, and global distribution (Reish and Gerlinger[1997]; Dean[2008]; Rhee et al.[2012]; Won et al.[2014]). However, the knowledge regarding the effects of diuron on marine polychaetes is still limited. Therefore, the objective of the present study was to evaluate the detrimental effects of diuron at sublethal concentrations through measurements of burrowing ability and AChE activity, following assessments of biochemical modulation of MDA and antioxidant defense enzymes (i.e., GSH, SOD, CAT, GR, and GPx) in the marine polychaete Perinereis aibuhitensis for 14 days. This information will contribute to our understanding of the ecotoxicological effects of diuron on benthic organisms and help assess the potential risks associated with its environmental exposure.
2. Materials and Methods
2.1 Marine polychaete
The marine polychaete P. aibuhitensis, with an average weight of approximately 1.45 ± 0.38 g and an age of 3 months after fertilization, were obtained from a polychaete aquaculture company located in Yeosu, Jeollanam-do, South Korea. The polychaetes were cultured under controlled laboratory conditions. The culture tanks used in the study were round polyethylene tanks that were opaque, ensuring minimal light penetration. The tanks were filled with filtered seawater, and the sieved sediment consisted of a mixture of sand and mud in an 8:2 ratio, which was supplied by the polychaete aquaculture company. The environmental conditions within the tanks were maintained at a constant photoperiod of 16 h light and 8 h dark, a temperature of 16 ± 0.7℃, a salinity of 33 practical salinity units, dissolved oxygen levels of 6.9 ± 0.5 mg L−1, and a pH of 8.0. To ensure proper water quality, 50% of the overlying filtered seawater in the tanks was replaced every two days. The polychaetes were provided with a diet consisting of ground TetraMarin® (Tetra, Blacksburg, VA, USA) at a rate of 0.1 g per individual, supplemented with a mixture of microalgae (1 × 106 cells mL−1) including Dunaliella sp., Isochrysis sp., and Tetraselmis sp. The feeding regimen was conducted three times weekly. Throughout the study, the salinity, temperature, dissolved oxygen, and pH levels of the culture tanks were monitored twice daily using a portable Orion Star meter (Thermo Fisher Scientific, Tewksbury, MA, USA).
2.2 Acute toxicity
For the experiments involving diuron exposure, diuron was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) obtained from the same supplier. Designated nominal concentrations of diuron were prepared by serial dilution of the stock solution in filtered seawater. To ensure the final DMSO concentrations remained within a range that had no detrimental effect on the polychaetes, the concentrations of DMSO ranged from 0.01% to 0.05% in the exposure volumes. Previous studies have shown that these DMSO concentrations did not have adverse effects on the polychaetes (Eom et al.[2019]; Haque et al.[2020]). To determine the LC50 value, thirty polychaetes were used, with 10 individuals per each concentration as triplicates. The experiments were conducted in opaque glass fiber tanks (dimensions: 55 × 45 × 36 cm; 21st-century HighTech®, Busan, South Korea) in the absence of sediment. The exposure conditions were the same as those used in the polychaete culture, including constant aeration with trickle flow. During the 96-hour exposure period to waterborne diuron, half of the test solution was renewed daily with the addition of an equivalent concentration of diuron. In the control group, half of the filtered seawater was renewed daily. No food was supplied to the polychaetes during the toxicity experiment. To determine the acute toxicity values, including the LC50 value, Probit analysis was performed using ToxRat® Professional 2.10.3.1 software (ToxRat Solutions GmbH, Alsdorf, Germany) based on the data obtained at the 96 h mark after exposure to diuron. Any dead polychaetes were promptly removed from the test chamber. Prior to the definitive test, a range-finding experiment was conducted using different concentrations of diuron, such as 1, 10, 100, 1000, and 5000 mg L−1. Based on the results of the range-finding experiment, the following concentrations of diuron were selected for the definitive test: 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 50, 500, and 1000 mg L−1.
2.3 Diuron exposure
For the evaluation of behavioral and biochemical responses of the marine polychaetes to sublethal concentrations of diuron, a total of approximately 600 polychaetes were collected. Twenty one polychaetes were divided into three sets as triplicate (N = 7 per replicate) and exposed to each treatment, including a control group, DMSO group, and three sublethal concentrations (1/50, 1/20, and 1/10 of the 96-hour LC50 value) of diuron (Table 1). The exposures were conducted in an opaque glass fiber tank (21st-century HighTech®) containing 9-10 cm of sediment. Sampling of the polychaetes was performed at various time points: 0 h, 24 h, 96 h, day 7, and day 14. The cultures were maintained under the same conditions as during the acclimation period. Out of the 21 polychaetes exposed to each treatment, 15 polychaetes were collected from the three sets, resulting in triplicates with 5 polychaetes per replicate. The five individuals per replicate were pooled and used for the analysis of biochemical responses. During the exposure period, half of the exposed solution was refreshed every 24 h with the addition of an equivalent concentration of diuron.
2.4 Burrowing ability
On day 14 of the experiment, the surviving polychaetes from each concentration of diuron were collected for a burrowing ability test. The methodology used for the burrowing assay followed previously established protocols for polychaete burrowing assessments (Bonnard et al. [2009]; Fonseca et al.[2018]; Haque et al.[2020]). Twenty polychaetes that survived from each treatment were transferred to clean filtered seawater in a 200 mL glass fiber container filled with sieved sediment, with a depth of 5 cm. The burrowing assay involved monitoring the number of fully burrowed polychaetes at two-minute intervals, with the monitoring period lasting for 30 minutes.
2.5 AChE activity
To determine the acetylcholinesterase (AChE) activity in the diuron-exposed polychaetes, the Ellman method (Ellman et al.[1961]) was employed with small modifications (e.g., buffer volume, basic instrument, and measuring device employed) adapted to a microplate reader. The following steps were performed: The pooled sample of polychaetes was homogenized using a Teflon homogenizer in 0.1 M phosphate buffer (pH 8.0). The homogenate was then centrifuged at 3,000 g for 30 minutes at 4℃, resulting in the separation of the supernatant. The separated supernatant was mixed with a cuvette containing 0.1 M phosphate buffer (pH 8.0), 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB, 0.01 M; Sigma-Aldrich, Inc.), and acetylthiocholine iodide (ATCh, 0.075 M; Sigma-Aldrich, Inc.). Blank solutions were prepared, one without ATCh and another without the sample, and they were tested alongside the samples. The mixture was incubated at 25℃ for 5 minutes, and the AChE activity was measured using a ThermoTM Varioskan Flash spectrophotometer (Thermo Fisher Scientific, Tewksbury, MA, USA) at an absorbance of 412 nm. To normalize the AChE activity, it was divided by the total protein content in the supernatant.
2.6 Measurement of antioxidant parameters
The measurement of MDA and antioxidant parameters in the diuron-exposed polychaetes was conducted following the methodology described in previous studies with P. aibuhitensis (Rhee et al.[2012]; Eom et al.[2019]; Haque et al.[2020]). The specific instructions provided by the manufacturer should have been followed. GSH concentration was measured in the pooled polychaete sample after washing in 0.9% NaCl using GSH Assay Kit (Catalog No. CS0260; Sigma–Aldrich, Inc.). The pooled sample was homogenized using a Teflon homogenizer and centrifuged at 10,000 × g for 10 minutes. Then, 10 μL of the supernatant was extracted and mixed with 150 μL of the Working Mixture in a 96–well plate. The mixture was incubated for 5 minutes at 25℃, followed by the addition of 50 μL of NADPH solution. The absorbance was measured by a spectrophotometer at a wavelength of 412 nm, and the GSH concentration was determined in μmol mL-1 using the GSH standard curve. Enzymatic activity of CAT was analyzed by CAT Assay Kit (Catalog No. CAT100; Sigma–Aldrich, Inc.). In brief, the pooled sample was homogenized using a Teflon homogenizer in phosphate buffer (50 mmol L-1, pH 7.0). The catalytic activity of the catalase enzyme in the supernatant was measured spectrophotometrically by monitoring changes in H2O2 absorbance at a wavelength of 240 nm at 25℃. Enzymatic activity of SOD was analyzed by SOD Assay Kit (Catalog No. 19160; Sigma–Aldrich Chemie, Switzerland), following the manufacturer's instructions. The pooled sample was homogenized in phosphate–buffered saline (50 mmol L-1, pH 7.4) containing 0.1 mM EDTA. Absorbance was measured at 450 nm to generate a curve and linearized rate. Enzymatic activities of GR and GPx were analyzed by GR Assay Kit (Catalog No. GRSA; Sigma–Aldrich, Inc.) and GPx Assay Kit (Catalog No. CGP1; Sigma–Aldrich, Inc.), respectively, following the manufacturer’s instructions. For GPx and GR activities, the pooled samples were homogenized in cold buffer (50 mM Tris–Cl, 5 mM EDTA, and 1 mM β–mercaptoethanol, pH 7.5) at a ratio of 1:4 (w/v) using a Teflon homogenizer. The homogenate was then centrifuged at 10,000 × g for 10 minutes at 4℃, and the upper aqueous layer containing the enzyme was collected for enzymatic assay. GPx and GR activities were measured by monitoring the absorbance at 340 nm. Enzymatic activities were expressed as units per milligram protein.
2.7 Statistic analysis
The statistical data analysis was performed using the statistical software package SPSS, Version 17 (SPSS Inc., Chicago IL, USA). A one-way analysis of variance (ANOVA) followed by a Duncan’s multiple range test was conducted to assess significant differences between the control group and the treated groups. The results of the analysis are presented as means ± standard deviation (S.D.) to indicate the central tendency and variability of the data, respectively. A P-value less than 0.05 was considered to indicate a significant difference between the groups.
3. Results and Discussion
3.1 Survival rate
The percentage of P. aibuhitensis that survived in response to different concentrations of diuron over a 96 h exposure period was measured (Fig. 1).
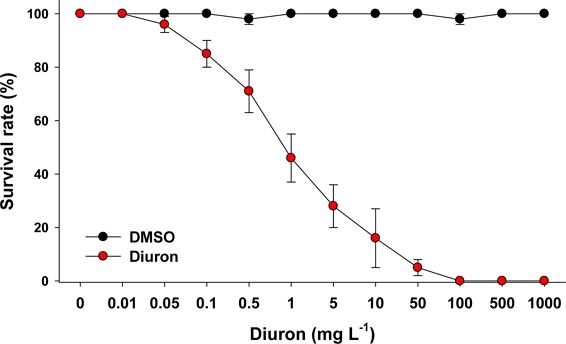
Measurement of the 96 h survival rate of the marine polychaete P. aibuhitensis in response to different concentration of diuron (0–1000 mg L–1). Data are presented as mean ± standard deviation.
In the control groups, the survival rate of P. aibuhitensis exceeded 97%. However, the mortality observed in the treated groups exhibited a clear dose-dependent and exposure duration-dependent pattern. The 96 h–LC50 value of diuron was calculated to be 0.48 mg L–1. At 96 h, there was a significant detrimental effect on survival at diuron concentrations above 0.1 mg L–1. Comparative data on diuron toxicity in other organisms are limited for polychaetes or oligochaetes. However, a previous study reported a 48 h–LC50 value of 16 mg L–1 for diuron in the serpulid tubeworm Hydroides elegans (Bao et al.[2011]). In fish species, the 96 h–LC50 values reported for diuron were 0.5 mg L–1 in striped bass Morone saxatilis (Hughes[1973]), 2.4 mg L–1 in Coho salmon Oncorhynchus kisutch (Mayer et al.[1986]), 6.7 mg L–1 in sheepshead minnow Cyprinodon variegatus (USEPA[2003]), and 7.8 mg L–1 in turbot Psetta maxima (Mhadhbi and Beiras[2012]). Based on the comparison of acute toxicity values, it can be concluded that P. aibuhitensis exhibits relatively higher sensitivity to diuron compared to other animals studied.
3.2 Burrowing ability and AChE activity
The number of unburrowed P. aibuhitensis individuals exposed to diuron exhibited a dose-dependent increase over a 30 min period (Fig. 2A). The highest concentration tested, which was 1/10 of the LC50 value for diuron, resulted in a substantial delay in burrowing activity. This delay in burrowing activity suggests a potential toxic effect of diuron on the basic bioturbation behavior and physiology of P. aibuhitensis. Although detailed physiological response was not measured, potential damage on muscle morphology, fluctuation in oxidative status and neural activity, and abnormal metabolism and energy production/consumption upon diuron would be the source of the harmful effect on the burrowing behavior of polychaetes (Dorgan et al.[2007]; Dorgan et al.[2008]). A similar delay in burrowing activity was observed in the same species when exposed to the pyrithione-based antifouling agent zinc pyrithione (ZnPT) (Haque et al.[2020]). This suggests that alternative antifoulants can have similar effects on the burrowing behavior of P. aibuhitensis.
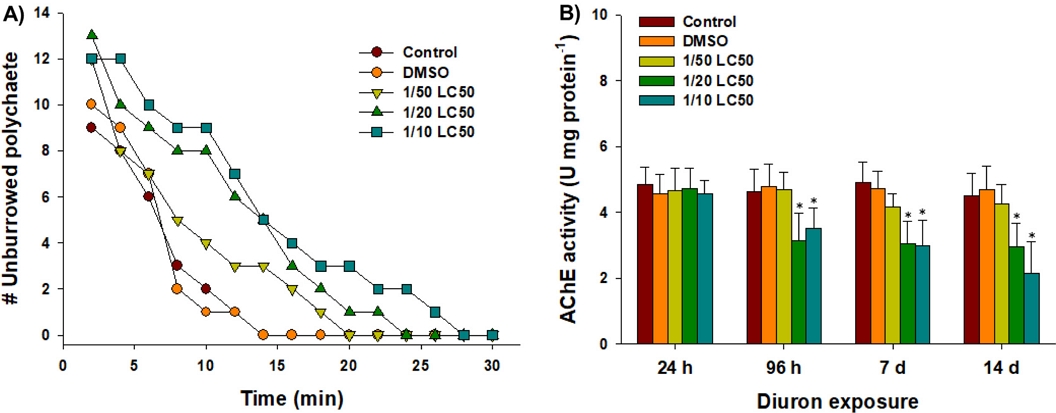
Changes in the (A) number of unburrowed polychaete and (B) acetylcholinesterase (AChE) activities in the marine polychaete P. aibuhitensis exposed to control, DMSO, and diuron (1/50, 1/20, and 1/10 of the LC50 values) for 30 minutes and 14 days, respectively. Data are presented as mean ± standard deviation (S.D.) of three replicates. Asterisk (*) on the data bar is indicating a significant difference (P < 0.05) compared to the control.
Significant inhibition of AChE activity was observed in P. aibuhitensis following exposure to 1/20 and 1/10 of the LC50 values of diuron at 96 h, day 7, and day 14, compared to the control group (P < 0.05) (Fig. 2B). This inhibition of AChE activity indicates that diuron interferes with the normal nerve communication in P. aibuhitensis. Maintaining homeostasis in GABAergic neurotransmission is crucial for the behavior and locomotion of polychaetes (Biscocho et al.[2018]). The inhibition of AChE can reduce the hydrolysis of acetylcholine (ACh) into choline, leading to an accumulation of ACh at synapses (Nunes[2011]). This accumulation of ACh can disrupt normal neurotransmission and potentially trigger neurotoxicity. In a study conducted on the polychaete H. diversicolor, exposure to 40 μg L–1 of the antifouling agent ZnPT for 96 h significantly inhibited AChE activity (Nunes and Costa[2019]). This finding supports the observed delayed burrowing activity in P. aibuhitensis exposed to diuron, indicating that the inhibition of AChE activity may be one of the mechanisms contributing to the toxic effects of diuron on the behavior of the polychaetes.
3.3 Oxidative stress and antioxidant response
To understand whether diuron is a modulator on oxidative status, oxidative markers and response of antioxidant components were measured in P. aibuhitensis. Significant increases in intracellular MDA content were observed in P. aibuhitensis exposed to 1/50, 1/20, and 1/10 of the LC50 values of diuron at 96 h, day 7, and day 14, compared to the control group (P < 0.05) (Fig. 3A). This finding indicates that diuron exposure leads to severe lipid peroxidation in the polychaetes. Reactive oxidants such as hydroxyl radicals, peroxides, superoxide, and singlet oxygen can cause damage to the carbon-carbon double bonds of polyunsaturated fatty acids in membrane lipids, leading to lipid peroxidation (Livingstone[2001]; Lesser[2006]). Analysis of lipid peroxidation, as measured by MDA content, is a well-established biomarker of oxidative stress. In a previous study, significant increases in intracellular MDA content were observed in P. aibuhitensis exposed to the antifoulant Sea-Nine (Eom et al.[2019]). Similarly, increased MDA content has been reported in several fish species exposed to diuron (Sánchez-Muros et al.[2013]; Sánchez-Muros et al.[2014]; Felicio et al.[2018]; Nam et al.[2023]). These findings indicate that diuron exposure induces oxidative stress and lipid peroxidation not only in P. aibuhitensis but also in other non-target animals, including fish species.
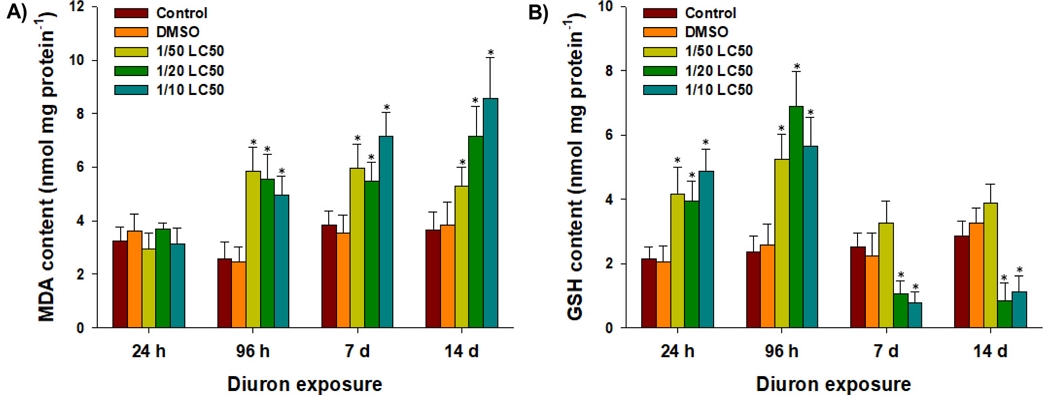
Changes in the activities of (A) malondialdehyde (MDA) and (B) glutathione (GSH) in the marine polychaete P. aibuhitensis exposed to control, DMSO, and diuron (1/50, 1/20, and 1/10 of the LC50 values) at 24 h, 96 h, day 7, and day 14. Data are presented as mean ± S.D., and an asterisk (*) on the data bar is indicating a significant difference (P < 0.05) compared to the control.
Significantly elevated glutathione (GSH) contents were detected in P. aibuhitensis exposed to 1/50, 1/20, and 1/10 of the LC50 values of diuron at 24 h and 96 h (P < 0.05) (Fig. 3B). However, significant GSH depletion was observed after exposure to 1/20 and 1/10 of the LC50 values at day 7 and day 14, compared to the control group (P < 0.05) (Fig. 3B). The elevated GSH levels observed in the early exposure period (24 hours and 96 hours) may indicate an adaptive response to neutralize or detoxify reactive oxidants and peroxides generated by diuron exposure. Increased GSH levels are typically associated with an organism’s defense against oxidative stress (Winston and Di Giulio[1991]; Lushchak[2011]). Similar findings have been reported in other organisms exposed to diuron. For example, zebrafish exposed to 5 and 10 mg L–1 of diuron showed an increase in intracellular GSH levels (Velki et al.[2019]). In red seabream, GSH levels significantly increased in response to 10 μg L–1 of diuron over a 60-day exposure period (Nam et al.[2023]). However, the observed depletion of GSH levels in P. aibuhitensis at day 7 and day 14 suggests either drastic GSH consumption or inhibition of GSH synthesis due to enhanced production of reactive oxidants by diuron. This finding indicates a potential disruption in the antioxidant defense mechanisms and an imbalance between oxidant production and GSH availability.
Taken together, the results on MDA and GSH levels indicate the oxidative potential of diuron and suggest a mutual correlation between lipid peroxidation and GSH depletion as consequences of oxidative stress in P. aibuhitensis. The organism’s initial adaptive response may be overwhelmed over time, leading to GSH depletion and increased lipid peroxidation.
3.4 Antioxidant defense system
In response to diuron exposure, there was an overall increase in antioxidant responses along with increased oxidative stress in P. aibuhitensis. CAT activity was significantly increased in polychaetes exposed to 1/50, 1/20, and 1/10 of the LC50 values of diuron at 24 h and 96 h (P < 0.05) (Fig. 4A). However, significantly decreased CAT activities were observed in response to 1/10 of the LC50 value at day 7 and day 14, and 1/20 of the LC50 value at day 14 (P < 0.05) (Fig. 4A). Similarly, SOD activity was significantly increased in response to 1/50 of the LC50 value at 96 h and day 7, and 1/20 and 1/10 of the LC50 values at 24 h and 96 h (P < 0.05) (Fig. 4B). However, significantly decreased SOD activities were measured after exposure to 1/10 of the LC50 value at day 7 and day 14, and 1/20 of the LC50 value at day 7 (P < 0.05) (Fig. 4B).
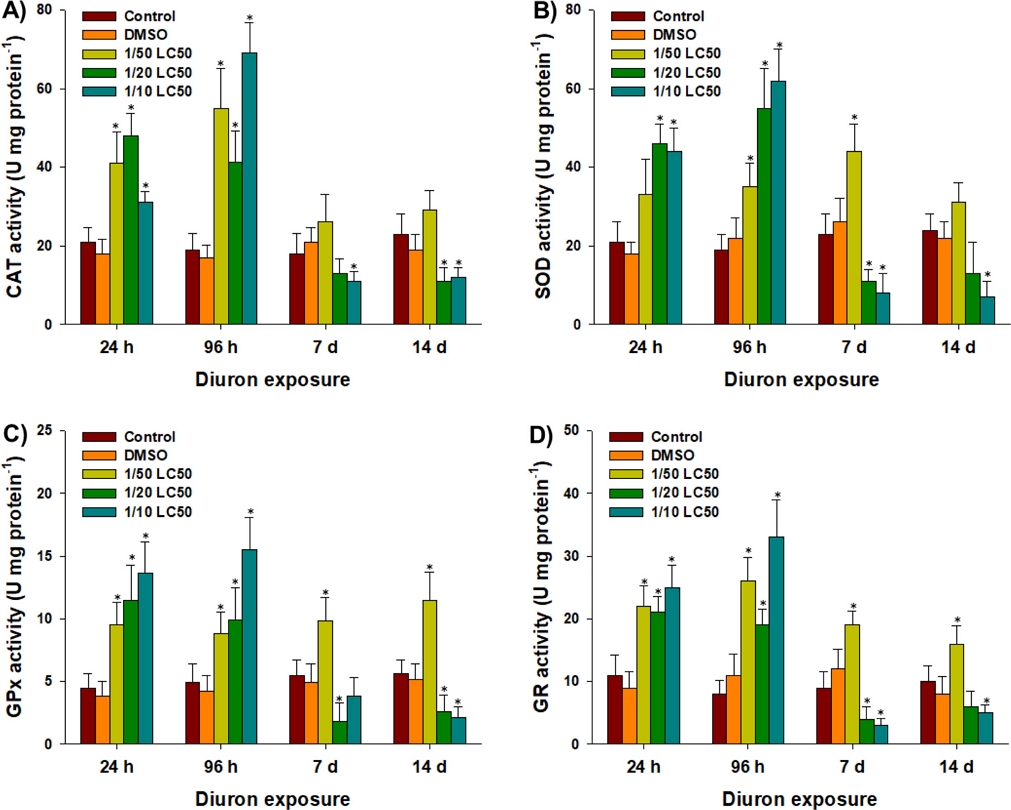
Changes in the activities of enzymes (A) catalase (CAT), (B) superoxide dismutase (SOD), (C) glutathione peroxidase (GPx), and (D) glutathione reductase (GR) in the marine polychaete P. aibuhitensis exposed to control, DMSO, and diuron (1/50, 1/20, and 1/10 of the LC50 values) at 24 h, 96 h, day 7, and day 14. Data are presented as mean ± S.D., and an asterisk (*) on the data bar is indicating a significant difference (P < 0.05) compared to the control.
Superoxide ion (O2•–) is a reactive oxidant that can be produced by xenobiotics and exogenous toxicants. SOD enzyme catalyzes the conversion of superoxide ion into O2 and H2O2 (Winston and Di Giulio[1991]; Lesser[2006]). To further detoxify H2O2, CAT enzyme converts it into H2O and O2 (Lushchak[2011]). This sequential association between SOD and CAT is an essential part of the organism's first line of antioxidant defense against oxidative stress (Winston and Di Giulio[1991]; Lushchak[2011]). The significant induction of CAT and SOD activities in response to relatively low concentrations of diuron observed during the early exposure period suggests an active response for the rapid elimination of reactive oxidants and oxidative stress. Similar findings have been reported in other species exposed to diuron. For example, gilthead sea bream exposed to 0.20 mg L–1 diuron for 60 days showed a significant increase in SOD activity (Sánchez-Muros et al.[2014]). Red seabream and black rockfish exposed to 1 and 10 μg L–1 diuron for 60 days also showed significant increases in CAT and SOD activities (Nam et al.[2023]). However, the significantly decreased activities of CAT and SOD observed at days 7 and 14 suggest the consistent accumulation of reactive oxidants, failure of their removal, and the induction of severe oxidative stress (Winston and Di Giulio[1991]; Lesser[2006]; Lushchak[2011]). These findings indicate that the antioxidant defense mechanisms become overwhelmed over time, leading to a compromised ability to counteract the reactive oxidants and maintain redox homeostasis.
The antioxidant components GPx and GR play crucial roles in reducing reactive oxidants, maintaining GSH levels, and minimizing oxidative stress in cells (Winston and Di Giulio[1991]; Lesser[2006]). In response to diuron exposure, GPx activity showed significant increases in polychaetes exposed to 1/20 and 1/10 of the LC50 values at 24 h and 96 h, and 1/50 of the LC50 value at all sampling points (P < 0.05) (Fig. 4C). However, significantly decreased GPx activities were observed in response to 1/20 of the LC50 value at day 7 and day 14, and 1/10 of the LC50 value at day 14 (P < 0.05) (Fig. 4C). Consistent with the patterns of CAT and SOD enzymes, GR activity showed significant increases in polychaetes exposed to 1/20 and 1/10 of the LC50 values at 24 h and 96 h, and 1/50 of the LC50 value at all sampling points (P < 0.05) (Fig. 4D). However, significantly decreased GR activities were observed in response to 1/20 of the LC50 value at day 7, and 1/10 of the LC50 value at day 7 and day 14 (P < 0.05) (Fig. 4D). GR enzyme is responsible for the reduction of glutathione disulfide (GSSG) to GSH, while GPx enzyme degrades H2O2 into H2O by utilizing GSH (Livingstone[2001]; Lesser[2006]). The elevated enzymatic activities of GPx and GR observed in relatively low concentrations of diuron-exposed polychaetes indicate their active involvement in the removal of reactive oxidants through conjugation with GSH, thereby minimizing oxidative stress. However, the significant decreases in enzymatic activities of GPx and GR in response to relatively high concentrations of diuron are consistent with the observed GSH depletion during the same exposure period. This suggests that the failure of GPx and GR activities directly affects the conjugation process with GSH, which can disrupt fundamental biological processes in marine polychaetes.
4. Conclusion
This study provides important insights into the potential effects of sublethal concentrations of the antifoulant diuron on a marine polychaete, shedding light on various aspects of its biology and physiology. The findings demonstrate that diuron can have detrimental effects on the behavior, cholinergic system, antioxidant defense system, and detoxification metabolism of the polychaete species, even though it is not the intended target of diuron. These results highlight the potential risks posed by environmentally relevant concentrations of diuron to nontarget aquatic organisms. Moving forward, it is crucial to consider the biodegradation of diuron in aquatic ecosystems and its bioaccumulation in animals in future research. Additionally, a mechanistic study focusing on the mode of action of diuron and its metabolites would provide a deeper understanding of how diuron exerts its effects on marine organisms. This knowledge can contribute to the development of more effective strategies for assessing the environmental impact of diuron and implementing appropriate mitigation measures.
Acknowledgments
This work was supported by the project titled “Techniques development for management and evaluation of biofouling on ship hulls–Korea Institute of Marine Science & Technology Promotion (KIMST–20210651)” funded by the Ministry of Oceans and Fisheries, South Korea.
References
-
Akcha, F., Barranger, A. and Bachère, E., 2020, Genotoxic and epigenetic effects of diuron in the Pacific oyster: in vitro evidence of interaction between DNA damage and DNA methylation, Environ. Sci. Pollut. Res., 28, 8266-8280.
[https://doi.org/10.1007/s11356-020-11021-6]

-
Bao, V.W., Leung, K.M., Qiu, J.W. and Lam, M.H., 2011, Acute toxicities of five commonly used antifouling booster biocides to selected subtropical and cosmopolitan marine species, Mar. Pollut. Bull., 62, 1147-1151.
[https://doi.org/10.1016/j.marpolbul.2011.02.041]

-
Behrens, D., Rouxel, J., Burgeot, T. and Akcha, F., 2016, Comparative embryotoxicity and genotoxicity of the herbicide diuron and its metabolites in early life stages of Crassostrea gigas: implication of reactive oxygen species production, Aquat. Toxicol., 175, 249-259.
[https://doi.org/10.1016/j.aquatox.2016.04.003]

-
Biscocho, D., Cook, J.G., Long, J., Shah, N. and Leise, E.M., 2018, GABA is an inhibitory neurotransmitter in the neural circuit regulating metamorphosis in a marine snail, Dev. Neurobiol., 78, 736-753.
[https://doi.org/10.1002/dneu.22597]

-
Bonnard, M., Romeo, M. and Amiard-Triquet, C., 2009, Effects of copper on the burrowing behavior of estuarine and coastal invertebrates, the polychaete Nereis diversicolor and the bivalve Scrobicularia plana, Hum. Ecol. Risk. Assess., 15, 11-26.
[https://doi.org/10.1080/10807030802614934]

-
Corrêa Mde, C., Maldonado, P., da Rosa, C.S., Lunkes, G., Lunkes, D.S., Kaizer, R.R., Ahmed, M., Morsch, V.M., Pereira, M.E. and Schetinger, M.R. 2008. Oxidative stress and erythrocyte acetylcholinesterase (AChE) in hypertensive and ischemic patients of both acute and chronic stages. Biomed. Pharmacother., 62, 317-324.
[https://doi.org/10.1016/j.biopha.2007.10.002]

-
Dahl, B. and Blanck, H., 1996, Toxic effects of the antifouling agent Irgarol 1051 on periphyton communities in coastal water microcosms, Mar. Pollut. Bull., 32, 342-350.
[https://doi.org/10.1016/0025-326X(96)84828-4]

- Dean, H.K., 2008, The use of polychaetes (Annelida) as indicator species of marine pollution: a review, Rev. Biol. Trop., 56, 11-38.
-
Dorgan, K.M., Arwade, S.R. and Jumars, P.A., 2007, Burrowing in marine muds by crack propagation: kinematics and forces, J. Exp. Biol., 210, 4198-4212.
[https://doi.org/10.1242/jeb.010371]

-
Dorgan, K.M., Arwade, S.R. and Jumars, P.A., 2008, Worms as wedges: effects of sediment mechanics on burrowing behavior, J. Mar. Res., 66, 219-254.
[https://doi.org/10.1357/002224008785837130]

-
Ellman, G.L., Courtney, K.D., Andres, Jr. V. and Feather-stone, R.M., 1961, A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol., 7, 88-95.
[https://doi.org/10.1016/0006-2952(61)90145-9]

-
Eom, H.-J., Haque, M.N., Nam, S.-E., Lee, D.-H. and Rhee, J.-S., 2019, Effects of sublethal concentrations of the antifouling biocide Sea-Nine on biochemical parameters of the marine polychaete Perinereis aibuhitensis, Comp. Biochem. Physiol. C, 222, 125-134.
[https://doi.org/10.1016/j.cbpc.2019.05.001]

-
Evans, S.M., Birchenough, A.C. and Brancato, M.S., 2000, The TBT ban: out of the frying pan into the fire?, Mar. Pollut. Bull., 40, 204-211.
[https://doi.org/10.1016/S0025-326X(99)00248-9]

-
Felício, A.A., Freitas, J.S., Scarin, J.B., de Souza Ondei, L., Teresa, F.B., Schlenk, D. and de Almeida, E.A., 2018, Isolated and mixed effects of diuron and its metabolites on biotransformation enzymes and oxidative stress response of Nile tilapia (Oreochromis niloticus), Ecotoxicol. Environ. Saf., 149, 248-256.
[https://doi.org/10.1016/j.ecoenv.2017.12.009]

-
Fonseca, T.G., Auguste, M., Ribeiro, F., Cardoso, C., Mestre, N.C., Abessa, D.M.S. and Bebianno, M.J., 2018, Environmental relevant levels of the cytotoxic drug cyclophosphamide produce harmful effects in the polychaete Nereis diversicolor, Sci. Total Environ., 636, 798-809.
[https://doi.org/10.1016/j.scitotenv.2018.04.318]

-
Giacomazzi, S. and Cochet, N., 2004, Environmental impact of diuron transformation: A review, Chemosphere, 56, 1021-1032.
[https://doi.org/10.1016/j.chemosphere.2004.04.061]

-
Haque, M.N., Nam, S.-E., Eom, H.-J., Kim, S.-K. and Rhee, J.-S., 2020, Exposure to sublethal concentrations of zinc pyrithione inhibits growth and survival of marine polychaete through induction of oxidative stress and DNA damage, Mar. Pollut. Bull., 156, 111276.
[https://doi.org/10.1016/j.marpolbul.2020.111276]

- Hughes, J.S., 1973, Acute toxicity of thirty chemicals to striped bass (Morone saxatilis). Pres. Western Assoc. State Game Fish. Comm. Salt Lake City, Utah.
-
Kaonga, C.C., Takeda, K. and Sakugawa, H., 2015, Antifouling agents and Fenitrothion contamination in seawater, sediment, plankton, fish and selected marine animals from the Seto Inland Sea, Japan, Geochem. J., 49, 23-37.
[https://doi.org/10.2343/geochemj.2.0327]

-
Kim, N.S., Shim, W.J., Yim, U.H., Hong, S.H., Ha, S.Y., Han, G.M. and Shin, K.H., 2014, Assessment of TBT and organic booster biocide contamination in seawater from coastal areas of South Korea, Mar. Pollut. Bull., 78, 201-208.
[https://doi.org/10.1016/j.marpolbul.2013.10.043]

-
Konstantinou, I.K. and Albanis, T.A., 2004, Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review, Environ. Int., 30, 235-248.
[https://doi.org/10.1016/S0160-4120(03)00176-4]

-
Lesser, M.P., 2006, Oxidative stress in marine environments: biochemistry and physiological ecology, Annu. Rev. Physiol., 68, 253-278.
[https://doi.org/10.1146/annurev.physiol.68.040104.110001]

-
Livingstone, D.R., 2001, Contaminated-stimulated reactive oxygen species production and oxidative damage in aquatic organisms, Mar. Pollut. Bull., 42, 656-666.
[https://doi.org/10.1016/S0025-326X(01)00060-1]

-
Lushchak, V.I., 2011, Environmentally induced oxidative stress in aquatic animals, Aquat. Toxicol., 101, 13-30.
[https://doi.org/10.1016/j.aquatox.2010.10.006]

-
Mayer, Jr., F.L., Mayer, K.S. and Ellersieck, M.R., 1986, Relation of survival to other endpoints in chronic toxicity tests with fish, Environ. Toxicol. Chem., 5, 737-748.
[https://doi.org/10.1002/etc.5620050804]

-
Mhadhbi, L. and Beiras, R., 2012, Acute toxicity of seven selected pesticides (alachlor, atrazine, dieldrin, diuron, pirimiphos-methyl, chlorpyrifos, diazinon) to the marine fish (turbot, Psetta maxima), Water Air Soil Pollut., 223, 5917-5930.
[https://doi.org/10.1007/s11270-012-1328-9]

-
Molochkina, E.M., Zorina, O.M., Fatkullina, L.D., Goloschapov, A.N. and Burlakova, E.B., 2005. H2O2 modifies membrane structure and activity of acetylcholinesterase. Chem. Biol. Interact., 157-158:401-404.
[https://doi.org/10.1016/j.cbi.2005.10.075]

-
Mukhtar, A., Zulkifli, S.Z., Mohamat-Yusuff, F., Harino, H. and Ismail, A., 2020, Distribution of biocides in selected marine organisms from South of Johor, Malaysia, Reg. Stud. Mar. Sci., 38, 101384.
[https://doi.org/10.1016/j.rsma.2020.101384]

-
Nam, S.-E., Haque, M.N., Do, S.D. and Rhee, J.-S., 2023, Chronic effects of environmental concentrations of antifoulant diuron on two marine fish: Assessment of hormone levels, immunity, and antioxidant defense system, Comp. Biochem. Physiol. C, 263, 109510.
[https://doi.org/10.1016/j.cbpc.2022.109510]

-
Nunes, B., 2011, The use of cholinesterases in ecotoxicology, Rev. Environ. Contam. Toxicol., 212, 29-59.
[https://doi.org/10.1007/978-1-4419-8453-1_2]

-
Nunes, B. and Costa, M., 2019, Study of the effects of zinc pyrithione in biochemical parameters of the Polychaeta Hediste diversicolor: evidences of neurotoxicity at ecologically relevant concentrations, Environ. Sci. Pollut. Res., 26, 13551-13559.
[https://doi.org/10.1007/s11356-019-04810-1]

-
Okamura, H., 2002, Photodegradation of the antifouling compounds Irgarol 1051 and Diuron released from a commercial antifouling paint, Chemosphere, 48, 43-50.
[https://doi.org/10.1016/S0045-6535(02)00025-5]

-
Perissini-Lopes, B., Egea, T.C., Monteiro, D.A., Vici, A.C., da Silva, D.G.H., Lisboa, D.C.D.O., de Almeida, E.A., Parsons, J.R., da Silva, R. and Gomes, E., 2016, Evaluation of Diuron tolerance and biotransformation by fungi from a sugar cane plantation sandy-loam soil, J. Agric. Food Chem., 64, 9268-9275.
[https://doi.org/10.1021/acs.jafc.6b03247]

- Reish, D.J. and Gerlinger, T.V., 1997, A review of the toxicological studies with polychaetous annelids, Bull. Mar. Sci., 60, 584-607.
-
Rhee, J.-S., Won, E.-J., Kim, R.-O., Choi, B.-S., Choi, I.-Y., Park, G.S., Shin, K.-H., Lee, Y.-M. and Lee, J.-S., 2012, The polychaete, Perinereis nuntia ESTs and its use to uncover potential biomarker genes for molecular ecotoxicological studies, Environ. Res., 112, 48-57.
[https://doi.org/10.1016/j.envres.2011.09.012]

-
Rico, E.P., Rosemberg, D.B., Dias, R.D., Bogo, M.R. and Bonan, C.D., 2007. Ethanol alters acetylcholinesterase activity and gene expression in zebrafish brain. Toxicol. Lett., 174, 25-30.
[https://doi.org/10.1016/j.toxlet.2007.08.005]

-
Saleh, A., Molaei, S., Fumani, N.S. and Abedi, E., 2016, Antifouling paint booster biocides (Irgarol 1051 and diuron) in marinas and ports of Bushehr, Persian Gulf, Mar. Pollut. Bull., 105, 367-372.
[https://doi.org/10.1016/j.marpolbul.2016.02.037]

- Sánchez-Muros, M.J., Trenzado Romero, C.E., Castillo, M.F., García Barroso, F. and Rus, A.S., 2014, Effect of low dose diuron in oxidative state on the gilthead sea bream Sparus aurata, Int. J. Aquat. Biol., 5, 130-144.
-
Sánchez-Muros, M.J., Villacreces, S., Miranda-de la Lama G., de Haro C. and García-Barroso F., 2013, Effects of chemical and handling exposure on fatty acids, oxidative stress and morphological welfare indicators in gilthead sea bream (Sparus aurata), Fish Physiol. Biochem., 39, 581-591.
[https://doi.org/10.1007/s10695-012-9721-2]

-
Sapozhnikova, Y., Wirth, E., Singhasemanon, N., Bacey, J. and Fulton, M., 2008, Distribution of antifouling biocides in California marinas, J. Environ. Monit., 10, 1069-1075.
[https://doi.org/10.1039/b806934d]

-
Schallreuter, K.U., Elwary, S.M., Gibbons, N.C., Rokos, H. and Wood, J.M., 2004. Activation/deactivation of acetylcholinesterase by H2O2: more evidence for oxidative stress in vitiligo. Biochem. Biophys. Res. Commun., 315, 502-508.
[https://doi.org/10.1016/j.bbrc.2004.01.082]

-
Schallreuter, K.U. and Elwary, S.M., 2007. Hydrogen peroxide regulates the cholinergic signal in a concentration dependent manner. Life Sci., 80, 2221-2226.
[https://doi.org/10.1016/j.lfs.2007.01.028]

-
Thomas, K., 2001, The environmental fate and behaviour of antifouling paint booster biocides: a review, Biofouling, 17, 73-86.
[https://doi.org/10.1080/08927010109378466]

-
Thomas, K.V., McHugh, M. and Waldock, M., 2002, Antifouling paint booster biocides in UK coastal waters: inputs, occurrence and environmental fate, Sci. Total Environ., 293, 117-127.
[https://doi.org/10.1016/S0048-9697(01)01153-6]

- USEPA, 2003., Reregistration Eligibility Decision for Diuron. Federal Register: Washington, DC, USA, 1-106.
-
Velki, M., Di Paolo, C., Nelles, J., Seiler, T.B. and Hollert, H., 2017, Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity, Chemosphere, 180, 65-76.
[https://doi.org/10.1016/j.chemosphere.2017.04.017]

-
Velki, M., Lackmann, C., Barranco, A., Ereno Artabe, A., Rainieri, S., Hollert, H. and Seiler, T.B., 2019, Pesticides diazinon and diuron increase glutathione levels and affect multixenobiotic resistance activity and biomarker responses in zebrafish (Danio rerio) embryos and larvae, Environ. Sci. Eur., 31, 1-18.
[https://doi.org/10.1186/s12302-019-0186-0]

-
Winston, G.W. and Di Giulio, R.T., 1991, Prooxidant and antioxidant mechanisms in aquatic organisms, Aquat. Toxicol., 19, 137-161.
[https://doi.org/10.1016/0166-445X(91)90033-6]

-
Won, E.-J., Ra, K., Kim, K.-T., Lee, J.-S. and Lee, Y.-M., 2014, Three novel superoxide dismutase genes identified in the marine polychaete Perinereis nuntia and their differential responses to single and combined metal exposures, Ecotoxicol. Environ. Saf., 107, 36-45.
[https://doi.org/10.1016/j.ecoenv.2014.03.026]

-
Yebra, D.M., Kiil, S. and Johansen, K.D., 2004, Antifouling technology – past, present and future steps towards efficient and environmentally friendly antifouling coatings, Prog. Org. Coat., 50, 75-104.
[https://doi.org/10.1016/j.porgcoat.2003.06.001]


